Macromolecular Proton Fraction (MPF): Quantitative Myelin Mapping Technique for Neuroscience
Applications
Brain development
 Figure 1. Example single-point brain MPF maps illustrating myelin development from the fetus to adulthood. MPF provides a uniform quantitative scale of myelination accross the lifespan.
Figure 1. Example single-point brain MPF maps illustrating myelin development from the fetus to adulthood. MPF provides a uniform quantitative scale of myelination accross the lifespan.
Myelination is a major morphological hallmark of the brain development reflecting physiological maturation of neural connectivity. Due to high sensitivity and specificity of MPF to myelination, this parameter provides a uniform quantitative scale of myelin development from the earliest pre-natal stage to the adulthood (Fig. 1). The fast MPF mapping method enables the design of rapid and versatile MRI protocols for studies of any stage of the brain development including fetuses and infants.
Fast fetal MPF mapping MPF mapping [1,2] demonstrated high sensitivity to the earliest stages of myelin formation. MPF values in the fetal brain appeared 4-6-fold lower as compared to white matter in the adult brain (2-3% vs. 12-13%), thus confirming that myelination provides the main determinant of MPF. MPF strongly correlated with gestational age (r range 0.7-0.9, P<0.001) in the brain structures with known prenatal onset of myelination, such as brain stem, cerebellum, and thalamus [1,2]. No significant age-related MPF changes were found in cerebral white matter, which is characterized by postnatal myelination onset [1,2].
MPF mapping became the primary tool for the studies of associations between brain structural maturation and the development of language and executive function in children from 6 months to adolescence carried out at the University of Washington Institute for Learning & Brain Sciences. Our collaborative publications [3-7] confirmed the utility of high-resolution 3D MPF mapping as both quantitative and structural imaging modality for a variety of human developmental neuroscience applications. The group templates created from individual MPF maps for adolescents [3] and 7- and 11-month-old infants [4] are presented in Fig. 2. The templates show exceptionally high brain tissue contrast, clear definition of fine anatomic structures, and anatomically consistent progression of myelination with age.
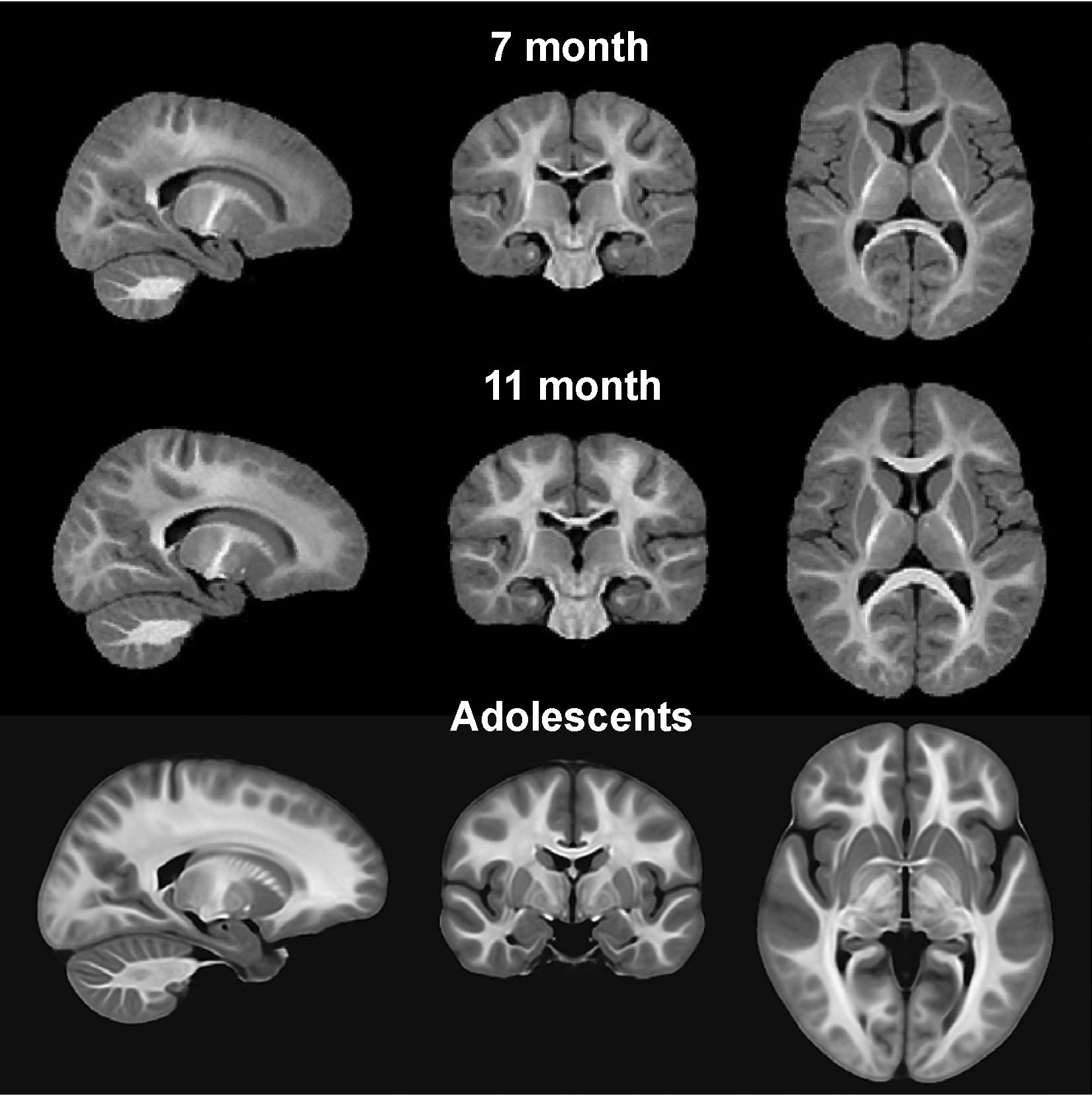 Figure 2. Group brain templates derived from high-resolution 3D MPF maps for 7-month-old infants, 11-month-old infants, and adolescents. The templates show unprecedented tissue contrast and clarity of anatomical details. Adapted from Refs. [3,4].
Figure 2. Group brain templates derived from high-resolution 3D MPF maps for 7-month-old infants, 11-month-old infants, and adolescents. The templates show unprecedented tissue contrast and clarity of anatomical details. Adapted from Refs. [3,4].
Neurologic and psychiatric diseases
 Figure 3. Example brain MPF maps obtained using the single-point method from a healthy person and multiple sclerosis (MS) patients with relapsing-remitting and secondary progressive disease courses.
Figure 3. Example brain MPF maps obtained using the single-point method from a healthy person and multiple sclerosis (MS) patients with relapsing-remitting and secondary progressive disease courses.
Myelin damage commonly occurs in numerous neurological conditions where it can be either a primary pathological substrate or a sequela of damage to axons, neurons, or oligodendroglia caused by neurodegenerative, traumatic, ischemic, toxic, infectious, and other injuries. The most common primary demyelinating disease, multiple sclerosis (MS) attracted significant interest as an area of clinical MPF applications. MS is typically associated with widespread myelin loss beyond MRI-visible lesions, which can be clearly perceived from MPF maps (Fig. 3). While the majority of earlier MS studies utilized more time-consuming multi-point quantitative MT techniques (see review [8] for details), the single-point method extended the potential of MPF to investigate demyelination in gray matter [9,10]. The first clinical study of fast MPF mapping in MS [9] demonstrated a profound MPF decrease in gray matter associated with the secondary progressive disease phenotype and identified strong correlation between gray matter MPF and disability. This finding emphasizes the primary clinical relevance of gray matter demyelination in MS, which is traditionally considered a white matter disease. Due to insensitivity of MPF to iron content changes in tissues, MPF mapping provides a unique capability to quantify demyelination in subcortical gray matter structures (such as globus pallidus, substantia nigra, putamen, etc.), which are frequently affected by excess iron deposition in MS [10] (Fig. 4).
Fast single-point MPF mapping also demonstrated promising results in the studies of mild traumatic brain injury study (mTBI) [11] and schizophrenia [12]. In mTBI, MPF maps revealed significant microscopic post-traumatic demyelination in normal-appearing brain tissues after blast injury with anatomical patterns consistent with the bio-mechanical mechanism of injury [11]. The schizophrenia study [12] demonstrated the capability of MPF to capture subtle hypomyelination associated with the disease duration and negative symptoms.
 Figure 4. Insensitivity of MPF to the brain tissue iron content illustrated in a case of relapsing-remitting MS. T2-weighted images identify excess iron deposition in the basal ganglia (top) and dentate nuclei (bottom). MPF maps clearly depict globus pallidus (GP, red arrow), putamen (blue arrow), and dentate nucleus (DN, green arrow) as gray matter structures. GP and DN are not identifiable on R1 maps and T1-weigted images. A small lesion in the left DN is seen only on the MPF map (yellow arrow).
Figure 4. Insensitivity of MPF to the brain tissue iron content illustrated in a case of relapsing-remitting MS. T2-weighted images identify excess iron deposition in the basal ganglia (top) and dentate nuclei (bottom). MPF maps clearly depict globus pallidus (GP, red arrow), putamen (blue arrow), and dentate nucleus (DN, green arrow) as gray matter structures. GP and DN are not identifiable on R1 maps and T1-weigted images. A small lesion in the left DN is seen only on the MPF map (yellow arrow).
Preclinical studies in animals
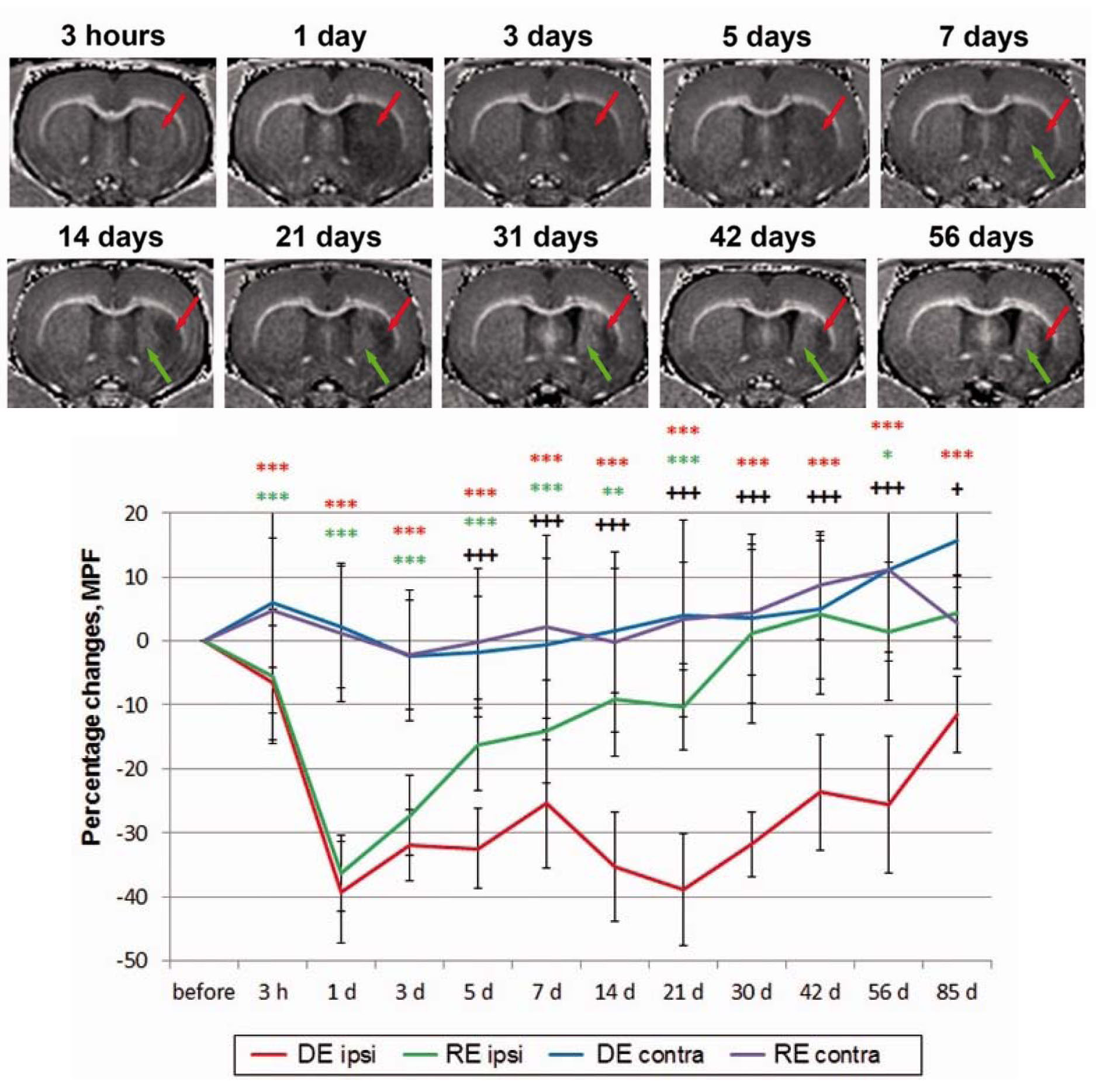 Figure 5. Longitudinal monitoring of the ischemic stroke lesion evolution in the rat middle cerebral artery occlusion model using MPF maps. MPF mapping identifies the subregions within the damaged tissue, which undergo either continuous demyelination (red arrow) or remyelination (green arrow) in the chronic stroke phase. The plot illustrates dynamics of the group mean percentage MPF changes relative to the baseline in the demyelination (DE) and remyelination (RE) zones and the corresponding contralateral locations. Adapted from Ref. [14].
Figure 5. Longitudinal monitoring of the ischemic stroke lesion evolution in the rat middle cerebral artery occlusion model using MPF maps. MPF mapping identifies the subregions within the damaged tissue, which undergo either continuous demyelination (red arrow) or remyelination (green arrow) in the chronic stroke phase. The plot illustrates dynamics of the group mean percentage MPF changes relative to the baseline in the demyelination (DE) and remyelination (RE) zones and the corresponding contralateral locations. Adapted from Ref. [14].
Fast MPF mapping provides a simple and versatile tool for pre-clinical animal studies of demyelination and remyelination. The method combines non-invasiveness of MRI and similar to histology accuracy of the myelin content assessment [8]. As such, MPF mapping enables longitudinal quantitative monitoring of myelination changes in the same animal, thus offering efficient study designs with a reduced number of animals, improved statistical power, and a reduction of the total cost of the experiment. The confirmed capability of MPF to monitor re-myelination [13,14] is of particular importance for pre-clinical testing of new therapeutic interventions targeted at myelin repair. An example of longitudinal changes in MPF associated with spontaneous processes of either protracted demyelination or remyelination in the ischemic stroke lesion [14] is illustrated in Fig. 5. An important advantage of MPF as a myelin biomarker is its independence of magnetic field strength [15,16]. This feature makes quantitative MPF-based effect estimates obtained using specialized high-field small-animal MRI equipment directly translatable to applications in humans utilizing lower-field clinical MRI systems.