Macromolecular Proton Fraction (MPF): Quantitative Myelin Mapping Technique for Neuroscience
Method
What is MPF?
 Figure 1. Two-pool model of the magnetization transfer (MT) effect in tissues.
Figure 1. Two-pool model of the magnetization transfer (MT) effect in tissues.
The term Macromolecular Proton Fraction (MPF) originates from the theory of the magnetization transfer (MT) effect. Classic MT theory [1,2] is based on the two-pool model, which treats tissue as a mixture of water and biological macromolecules (Fig. 1). Protons of water and macromolecules form the two pools with distinct magnetic resonance properties. Water protons rapidly reorient in the space (liquid phase) and have relatively long T2 (tens of milliseconds). They mainly produce the signal observable in MRI. Macromolecular protons have restricted motion and behave like solid materials (semi-solid phase) with very short T2 (tens of microseconds). It is difficult or even impossible to observe their signal directly. Water and macromolecular protons are frequently referred to the literature as the “free pool” and “bound pool,” respectively. Once nuclear magnetization has been created in such a two-pool system by a radiofrequency (RF) pulse, it evolves through several pathways. Within each pool, magnetization is rapidly distributed via either diffusion (in water) or spin-diffusion (in macromolecules). The later process is defined as relayed transfer of magnetic energy through a series of dipolar interactions across backbone chains of biopolymers. The MT effect is caused by exchange of magnetic energy between the pools at their interface. There are two mechanisms involved: cross-relaxation (dipolar interaction between closely positioned protons, main effect) and chemical exchange (migration of a proton from one molecule to another, a relatively minor effect). Regardless of the mechanism, magnetization exchange at the water-macromolecule interface is much slower compared to the equilibration processes within each phase. Accordingly, only a single exchange process can be taken into account to describe the MT effect.
MPF is defined as a relative amount (in %) of macromolecular protons involved into the MT effect:

where [Hm] and [Hw] are the absolute concentrations of macromolecular and water protons [3].
Based on the above definition of MPF, two physical consequences are practically important:
- MPF is related to the concentrations of protons but not the whole macromolecules;
- MPF is not related to all macromolecular protons. It reflects only a portion of macromolecular protons that exhibits solid-like molecular dynamics. This behavior is typically observed in large supermolecular assemblies with liquid-crystal properties, such as myelin [4,5] and collagen [1,6].
Fast MPF Mapping
 Figure 2. Magnetization dynamics in the two-pool model of the MT effect.
Figure 2. Magnetization dynamics in the two-pool model of the MT effect.
Generally, the dynamics of magnetization in the two-pool is quite complex (Fg. 2) and requres 7 parameters for its mathematical description (total equilibrium magnetization or proton density (PD), MPF, cross-relaxation rate constant, relaxation times T1 and T2 of each pool). The fast single-point MPF mapping method [3] enables reconstruction of MPF maps in isolation from other two-pool model parameters based on a single MT-weighted image. The principle of the method is based on the iterative solution of the pulsed MT matrix equation [3,7] with respect to the parameter of interest (MPF) using appropriate constraints for other model parameters. The single-point method [3] exploits negligible variability of the cross-relaxation rate constant R defined for the bound-to-free pool transfer (Fig. 2), T2 of macromolecular protons, and the product of observed R1=1/T1 and T2 of the free pool in brain tissues.
Further acceleration of the single-point technique has been achieved by elimination of a reference image [8], exclusion of B0 mapping due to a negligible effect of B0-related errors [9], and a data-driven algorithm for B1 non-uniformity correction [10]. The last algorithm reconstruct a surrogate B1 map based on an algebraic relationship between errors caused by B1 inhomogeneity in R1 and MPF. Surrogate B1 correction obviates the need for B1 mapping sequences, which are typically unavailable on clinical MRI systems. In the current state of development, the entire single-point MPF mapping protocol consists of only three spoiled 3D gradient-echo sequences providing MT-, T1-, and PD-weighted images (Fig. 3). These sequences are available for all commonly used clinical MRI platforms and can be implemented without modification of original scanners' software. Additionally, the method produces R1, (or T1), and PD maps (Fig. 3). Fast MPF mapping provides excellent reproducibility with the coefficients of variation for repeated MPF measurements less than 2% in the human [9] and animal [5] brain.
 Figure 3. Example input images (top) and output maps (bottom) produced by the fast MPF mapping method. Adapted from Ref. [8].
Figure 3. Example input images (top) and output maps (bottom) produced by the fast MPF mapping method. Adapted from Ref. [8].
MPF as a myelin biomarker
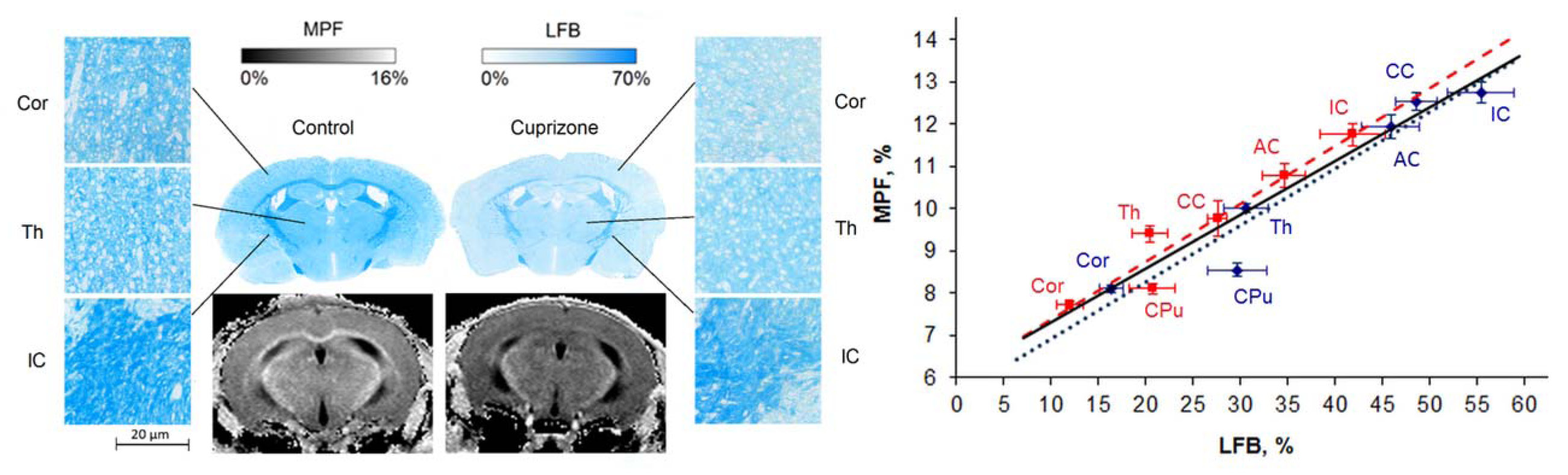 Figure 4. Example histological sections stained with luxol fast blue (LFB) and MPF maps of the control and cuprizone-treated mice (left) and correlation between MPF and LFB density across anatomic structures (right) including the corpus callosum (CC), anterior commissure (AC), internal capsule (IC), caudoputamen (CPu), thalamus (Th), and cortex (Cor). Red and blue colors correspond to the cuprizone-treated and control groups, respectively. Adapted from Ref. [5].
Figure 4. Example histological sections stained with luxol fast blue (LFB) and MPF maps of the control and cuprizone-treated mice (left) and correlation between MPF and LFB density across anatomic structures (right) including the corpus callosum (CC), anterior commissure (AC), internal capsule (IC), caudoputamen (CPu), thalamus (Th), and cortex (Cor). Red and blue colors correspond to the cuprizone-treated and control groups, respectively. Adapted from Ref. [5].
MPF attracted a substantial interest as a myelin biomarker, which demonstrated strong linar correlations with the myelin content in a number of animal model studies (see review [11] for details). Several recent studies specifically focused on histological and immunohistochemical validation of the fast single-point MPF mapping method [5,12-15]. This method was comprehensively validated in the animal models of toxic reversible demyelination induced by cuprizone [5,12], ischemic stroke [13,14], and early post-natal brain development [15].
In the murine cuprizone demyelination model (Fig. 4), MPF demonstrated the capability to identify demyelination not only in white matter (p<0.001 for corpus calosum), but also in both cortical and subcortical gray matter (p=0.005 and 0.01 for the cortex and caudate putamen) [5]. MPF values across anatomical structures strongly correlated with quantitative LFB histology in both the animal groups taken separately (r=0.96, p<0.002 for the treatment group and r=0.93, p=0.007 for the control group) and combined sample (r=0.95, p<0.001) with nearly identical regression coefficients, thus indicating that MPF provides a uniform measure of the myelin content in both normal and demyelinated tissues (Fig. 4, [5]). In the study of re-myelination after cuprizone withdrawal [12], MPF demonstrated close agreement with MBP immunohistology in demyelination, re-myelination, and control groups. MPF and MBP-stained area strongly correlated in all studied anatomic structures (corpus callosum, caudate putamen, hippocampus, and cortex; r range 0.80–0.90, p<0.001).
Using a rat stroke model, MPF and other quantitative MRI parameters were compared with histological markers of demyelination (LFB), neuronal loss, axonal loss, and inflammation in the acute and sub-acute stroke phases [13]. Demyelination develops as early as on the first day that is in agreement with histological evidence from earlier studies. LFB density strongly correlated with MPF in the ischemic lesion (r=0.81, P<0.001) and did not correlate with other MRI parameters, such as T1, T2, PD, and diffusion coefficient. MPF also did not correlate with histological measures of neuronal and axonal density and inflammation, thus confirming specificity to myelin. A longitudinal study in the rat stroke model [14] histologically confirmed the unique capability of MPF to detect sub-regions of continuous demyelination and re-myelination during evolution of the chronic ischemic stroke lesion. The remyelination zones exhibited active axonal regrowth, reconstitution of compact fiber bundles, and proliferation of neuronal and oligodendroglial precursors. Notably, astroglial scarring in the chronic stroke lesion identified by GFAP immunofluorescence appeared coincident with a zone of profound myelin loss characterized by low MPF and weak LFB or MBP staining.
In the study of the postnatal brain development in rabbits [15], MPF temporal trajectories showed a period of fast growth during the first two postnatal weeks quantitatively consistent with MBP western blot and electron microscopy. During the pre-myelination stage, MPF was very low (between 2 and 3%) in both white and gray matter. Strong correlations were observed between MPF and MBP content with r=0.9 (P<0.001) in white matter structures (internal capsule and corpus callosum) and r=0.79-0.93 (P<0.001) in cortical gray matter regions. Myelin fraction estimated by electron microscopy also strongly correlated with MPF in the internal capsule (r=0.86, P<0.001) and corpus callosum (r=0.95, P<0.001).
Collectively, the results of the above histological validation studies demonstrate high sensitivity and specificity of MPF to the myelin contend changes caused by demyelination, remyelination, and normal myelin development. Importantly, MPF is unaffected by the majority of concomitant pathological changes in brain tissues, such as neuronal and axonal loss, microglial proliferation, and astrogliosis.
References
- Edzes HT, Samulski ET. Measurement of cross-relaxation effects in proton NMR spin-lattice relaxation of water in biological-systems - hydrated collagen and muscle. J Magn Reson. 1978;31(2):207–229.
- Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR Biomed. 2001;14(2):57-64.
- Yarnykh VL. Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn Reson Med 2012;68(1):166-178.
- Underhill HR, Rostomily RC, Mikheev AM, Yuan C, Yarnykh VL. Fast bound pool fraction imaging of the in vivo rat brain: Association with myelin content and validation in the C6 glioma model. Neuroimage 2011;54(3):2052-2065.
- Khodanovich MY, Sorokina IV, Glazacheva VY, Akulov AE, Nemirovich-Danchenko NM, Romashchenko AV, Tolstikova TG, Mustafina LR, Yarnykh VL. Histological validation of fast macromolecular proton fraction mapping as a quantitative myelin imaging method in the cuprizone demyelination model. Sci Rep 2017;7:46686.
- Yarnykh VL, Tartaglione EV, Ioannou GN. Fast macromolecular proton fraction mapping of the human liver in vivo for quantitative assessment of hepatic fibrosis. NMR Biomed 2015;28(12):1716-1725.
- Yarnykh VL. Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human MRI: theory and clinical applications. Magn Reson Med 2002;47(5):929-939.
- Yarnykh VL. Time-efficient, high-resolution, whole brain three-dimensional macromolecular proton fraction mapping. Magn Reson Med 2016;75(5):2100-2106.
- Yarnykh VL, Kisel AA, Khodanovich MY. Scan-Rescan Repeatability and Impact of B(0) and B(1) Field Nonuniformity Corrections in Single-Point Whole-Brain Macromolecular Proton Fraction Mapping. J Magn Reson Imaging 2020;51(6):1789-1798.
- Yarnykh VL. Data-driven Retrospective Correction of B1 Field Inhomogeneity in Fast Macromolecular Proton Fraction and R1 Mapping. IEEE Trans Med Imaging 2021;40(12):3473-3484.